Lab work, discovering the whole iceberg
Lab work, discovering the whole iceberg
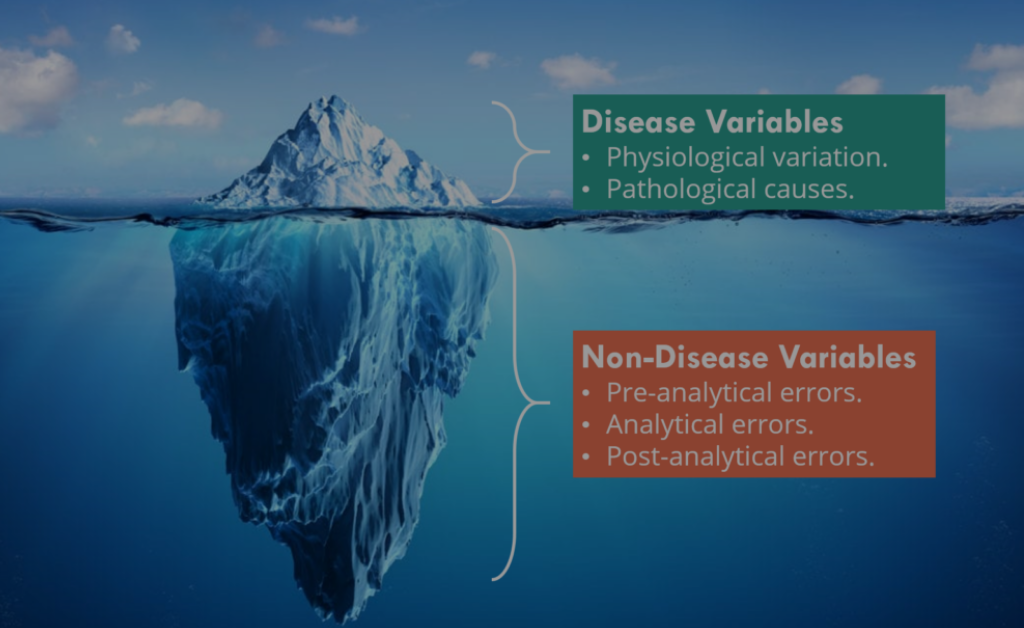
A laboratory result is like an iceberg, you should always pay attention to the hidden part!
When it comes to laboratory investigation, there are numerous caveats to take care of. Laboratory results should not be interpreted in isolation, but with an understanding of the laboratory methods used and the potential errors caused by inappropriate sample collection and handling.
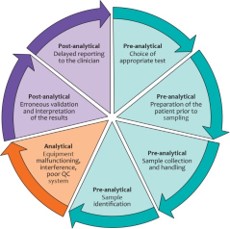
Errors may be introduced into the diagnostic laboratory cycle at three main stages:
- Pre-analytical errors.
- Analytical errors.
- Post-analytical errors.
Sources of potential errors
- Pre-analytical errors
- Most errors affecting laboratory Testing occur in the preanalytical phase.
- Poor quality or inappropriate samples can lead to the generation of poor quality results, which can cause erroneous clinical interpretation, resulting in poor patient care.
- Preanalytical errors can be sub-classified as follows:
a) Choosing the right parameters.
b) Preparation of the patient before sampling, and patient variables.
c) Sample collection and handling.
d) Problems with identification.
A) Choosing the right parameters.
- When choosing a laboratory test, the clinician should consider sensitivity, specificity, and predictive value of the test for identifying the suspected disease:
- Tests with high sensitivity are mainly used to rule out suspected diseases.
- Tests with high specificity are used to rule out suspected diseases.
- The clinician also should consider using single parameters Vs ready-to-use profiles.
B) Preparation of the patient before sampling, and patient variables
- The most common physiological changes or patient variables that can affect some test results are:
- Exercise or excitement/fear can cause changes in some haematology parameters due to the release of catecholamines (physiological leucocytosis).
- Stress secondary to animal handling or underlying disease may have profound effects on laboratory results, due to endogenous corticosteroid release (e.g. stress leucocytosis), every effort should be taken to minimize stress during blood sample collection.
- Food consumption can affect biochemistry tests, in particular cholesterol, triglyceride, glucose and urea, additionally, a postprandial sample may be lipemic and this can be depending on the analytical method, affect other biochemical tests (total proteins, electrolytes, haematology parameters).
- Drugs can interfere with the measurement of the analyte either in vivo (due to effects of the drug or metabolites on the analyte to be measured), or in vitro (effects due to some physical or chemical property of the drug or its metabolites which interfere with the actual assay procedure).
- If samples are to be collected for monitoring drug therapy/effect (e.g. thyroid supplementation, glucose tolerance curve), collection times can be important and should be carefully recorded.
- Additionally, there are some variables intrinsic to the patient that, if ignored, could lead to incorrect interpretation of the results, the more common patient variables are species, breed, age & sex-related.
C) Sample collection and handling
- Incorrect sample collection and handling can lead to an unsuitable sample for analysis, potentially leading to inaccurate results and incorrect clinical decision.
- The most common reasons why a sample may not be suitable
for analysis are: - Incorrect sampling technique: for example, urine for microbiology testing should be collected aseptically by
cystocentesis, while for routine urinalysis an uncontaminated voided sample collected into a clean container is often appropriate, urine from the cage floor is unsuitable for any analysis. - Clotted sample (micro and macro clots): traumatic or delayed blood collection can cause platelet activation and secondary aggregation, leading to spurious thrombocytopenia, it may also falsely decrease the white blood cell (WBC) count.
- Hemolysis: venipuncture should be performed rapidly and as atraumatically as possible to reduce the potential for hemolysis. parameters that are more affected include haematology parameters, creatine kinase (CK), aspartate aminotransferase (AST), phosphate and total protein (due to absorbing the same
wavelength as the coloured product of a reaction, or the substance being measured is released from lysed red cells). - The choice of anti-coagulant: the anticoagulant can affect the results of testing.
- Under- or over-filling of blood tubes: tubes should be filled to the correct volume and gently inverted to mix the blood with the pre-measured contents. it is important to collect an adequate volume of blood for the tests required.
- At the pre-analytical stage, the greatest numbers of errors for haematology tests are introduced by the ageing of the sample.
- Samples for measurement of ionized calcium and magnesium must also be handled carefully. serum should be separated quickly and stored anaerobically.
- Samples for glucose determination need to be separated promptly or placed in fluoride/oxalate because glucose decreases at a rate of 10% per hour if unseparated samples are held at room temperature.
D) Problems with identification
Examples of problems with the identification of the sample are:
- Specimens not labelled or incorrectly labelled
- A mismatch between the sample’s label and the submission form
- Incorrect information provided on the submission form.
2. Analytical errors
a) Features inherent to the sample (interferences):
1) Hemolysis:
- Visual: sample has a transparent reddish hue, ranging from pale pink to deep red.
- Indications: intravascular hemolysis or damage to RBCs during sample preparation.
- Lab artefacts: Lipemia alters the light-scattering property of blood, causing alterations of a variety of hematologic and chemical results.
2) Lipemia:
- Visual: sample has a pale, milky appearance, possibly with floating fat globules.
- Indications: recent ingestion of a fatty meal or dysfunction in fat metabolism.
- Lab artefacts: hemolysis releases free haemoglobin that may interfere with spectrophotometric assays that measure substances at wavelengths similar to the absorbance range of haemoglobin. it also falsely raises MCHC and lowers the packed cell volume (PCV) and the red cell count.
3) Icterus:
- Visual: plasma has a transparent yellow to opaque brown color.
- Indications: intravascular hemolysis, toxic or obstructive liver diseases.
- Lab artefacts: bilirubin affects creatinine and total protein concentrations, both of which are decreased with high bilirubin concentrations
- When patients are sick, there’s no time for second-guessing or incomplete results.
- Up to 73% of blood samples in veterinary medicine can be affected by interfering substances; this means that nearly 3 out of 4 veterinary blood samples can be compromised by hemolysis, lipemia, or icterus.
b) Features inherent to the analyzer (assay performance):
The accuracy & precision of assay performance is dependent upon the method used by the analyzer and quality control systems.
b)Features inherent to the analyzer (assay performance):
- The accuracy & precision of assay performance is dependent upon the method used by the analyzer and quality control systems.
What does IDEXX have to offer?
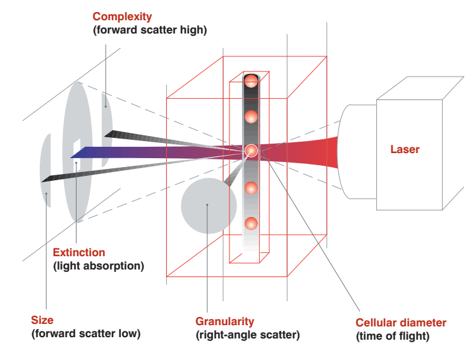
- IDEXX LaserCyte Dx & IDEXX ProCyte One use laser flow cytometry which is a far superior technology compared to impedance analyzers.
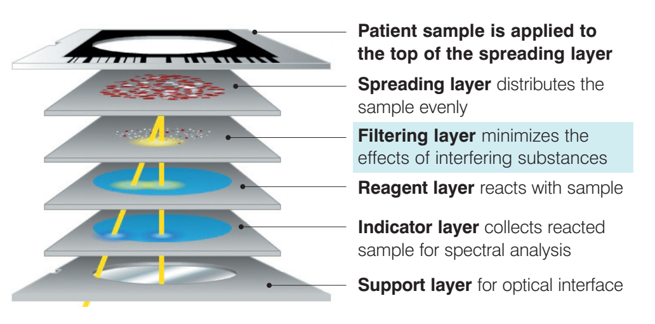
- IDEXX Catalyst DX & IDEXX Catalyst One use dry chemistry & dry slide technology to filter out any interferences.
- IDEXX LaserCyte has a great advantage over impedance counting because not only size but also cell complexity/density is used to distinguish cell types.
- Platelets and red cells are more accurately distinguished; because red cells are packed with haemoglobin they are much denser than platelets.
- In a retrospective data analysis using random Catalyst One analyzers (n = 255,456 samples), the Catalyst One Chemistry Analyzer provided complete results 98.1% of the time, suppressing results only 1.9% of the time due to interfering substances.
- Compromised blood samples on other analyzers can affect your test results when it matters most!
3. Post-analytical errors
Errors can occur as a result of:
- Reporting incorrect values/units.
- Assigning the results to the wrong patient.
- Providing the incorrect reference values for the species may be provided.
- However, the majority of errors at this stage are related to the interpretation of the results; here are some of the pitfalls in diagnostic testing that clinicians must be aware of:
- Laboratory results should be interpreted in the light of the available clinical data;
- The meaning of a normal range: normal ranges are established to include 95% of the healthy population; this
means that 5% of the population would be outside the normal range, and could be misdiagnosed as having a disease; - Tests are rarely 100% sensitive: this means that an animal
could have a disease and the test would not detect it; - Tests are rarely 100% specific: this means that the test may say
the animal has the disease when in fact it doesn’t ; - Many tests have a grey zone: this means that there can be
overlap between what is considered normal and abnormal.
Recent Newsletter
| M | T | W | T | F | S | S |
|---|---|---|---|---|---|---|
| 1 | ||||||
| 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| 16 | 17 | 18 | 19 | 20 | 21 | 22 |
| 23 | 24 | 25 | 26 | 27 | 28 | 29 |
| 30 | ||||||